Does COVID-19 Pandemic triggers compulsory licensing?
COVID-19 & INDIA
India, second in the list of worst affected countries by COVID-19. Approximately, 10,880,603 confirmed cases and 155,447 deaths have been occurred till date. When we, compare on the basis of deaths, then India holds fourth position. In last 24 hours, approx. 10k new cases have been confirmed.
India is second heavily populated country in the world after china. It will be very difficult and challenging task for government to ensure successful vaccination of its citizens. The nationwide drive was inaugurated by The Prime Minister Narendra Modi on January 16, with priorities given to healthcare and frontline workers in the first phase. From 13 Feb, second shot of vaccine began in India.
Indian Vaccine Landscape
There are 9 COVID-19 vaccine candidates in India. Out of these candidates, only Oxford Astrazeneca's Covishield & Bharat Biotech's Covaxin are currently used for inoculation in India. The Indian government has ordered 10 million Covaxin doses and 21 million AstraZeneca shots. Both vaccines have to be stored at 2°C to 8°C to maintain potency and efficacy, this temperature range is more practical to maintain in comparison to Moderna & Pfizer-BioNtech vaccines storage temperature range that is -20°C to -80°C. We can say Covaxin & ZyCoV-D as "Swadeshi Vaccine" for Covid-19 because they are of fully Indian origin.
Covishield- Serum Institute of India, the Indian makers of the vaccine, say vaccine is highly effective and backed by phase lll trial data from Brazil and United Kingdom. But patients' rights group, All India Drug Action Network, says its approval has been rushed because the manufacturer has not completed a "bridging study" of the vaccine on Indians. The company has said it will try to conduct the bridging trail of the vaccine in India in February.
Covaxin - India's first homemade vaccine. One notable point is Covaxin had been approved for "restricted use in emergency situations in public interest as an abundant precaution, in clinical trial mode, especially in the context of infection by mutant strains". There are some important concerns about the safety and efficacy of this vaccine because it was approved without Phase lll clinical trials data. The All India Drug Action Network (AIDAN) has asked to Indian government to withdraw the approval of Covaxin. However, Bharat Biotech has defended the approval, saying Indian clinical trial laws allowed "accelerated" authorization for use of drugs after the second phase of trials for "unmet medical needs of serious and life-threatening diseases in the country". It has promised to provide efficacy data for the vaccine by the end of Feb. Let's hope for favorable phase lll data.
ZyCoV-D -Phase l/ll clinicals trials data of ZyCoV-D indicated that the vaccine is safe and it has been approved by Drugs Controller General of India (DCGI), for the conduct of Phase lll clinical trials.
The Regulator is expected to approve Russia's Sputnik V and Cadila Healthcare ZyCov_D vaccines in next few months.
Some of the COVID-19 vaccines compared
Is it time to grant "Compulsory License" ?
India and South Africa on 2nd October 2020, asked the World Trade Organization to allow all countries to choose nor grant neither enforce patent and other IPs related to COVID drugs, Vaccines, Diagnostics and other technologies during pandemic (Temporary International Waiver) until, global herd immunity is achieved. Unfortunately, Some WTO members opposed this proposal and traditionally backed the interests of Pharmaceutical Corporation through a proprietary IP system. Recently 14 members of the European Parliament (MEPs) backed above joint proposal and said about granting of a compulsory licensing for the production of COVID 19 vaccines, according to TRIPS and Doha Declaration.
Compulsory Licensing - When a government allows someone else to produce patented products without the consent of the patent owner or plans to use patent -protected inventions itself. It is one of flexibilities in the field of patent protection included in the WTO's agreement on IPR - the TRIPS. It is not as same as tearing up the patent. The patent owner still has rights and they get compensation for copies of the products made under the compulsory licensing.
 In March 2012, India granted its first compulsory license ever. The license was granted to Indian drug manufacturer Natco Pharma for Sorafenib Tosylate, a cancer drug "Nexavar" patented by Bayer Corporation.
In March 2012, India granted its first compulsory license ever. The license was granted to Indian drug manufacturer Natco Pharma for Sorafenib Tosylate, a cancer drug "Nexavar" patented by Bayer Corporation.
Laws & Rules for Compulsory licensing
Under the Article 5A (2) of Paris Convention (1883) (2), Each country of the Union shall have the right to take legislative measures providing for the grant of compulsory licenses to prevent the abuses which might result from the exercise of the exclusive rights conferred by the patent.
Under the TRIPS (1995) Article 30 provides limited exceptions to the rights conferred under patents so that unreasonable prejudice to the legitimate interests of the patent owner is not done. This article also provides for power to issue compulsory licenses.
‘Compulsory license’ was specifically mentioned under Chapter XVI and conditions to be fulfilled are laid down under section 84 to 92 of the Indian Patents Act, 1970
On 1st January 2005 the Indian Patent Act was amended to introduce section 92 (A) to grant compulsory licenses for pharmaceutical products. At any time after the expiration of three years from the date of the grant of a patent, any person interested may make an application to the Controller for grant of compulsory license on patent on any of the following grounds, namely
(a) That the reasonable requirements of the public with respect to the patented invention have not been satisfied, or
(b) That the patented invention is not available to the public at a reasonably affordable price, or
(c) That the patented invention is not worked in the territory of India.
Section 92 – Special provision for compulsory licenses on notifications by the Central government.
Section 92 (3) – Scenarios of national emergency or extreme urgency, a case of public non-commercial use and for exports of patented pharmaceutical products in certain exceptional circumstances.
This pandemic has exposed fundamental flaws in the current system of medical innovation and access to medicine, which require urgent attention from global community. There is a significant concern that the current IP protection and manufacturing capacities may become a barrier to access COVID medicines/vaccines. Because these medicines/vaccines will be distributed to billions of people worldwide. Pharmaceutical companies are increasing their manufacturing capacities but this may not be enough for adequate allocation. As a result, only some countries will be the first to access these medicines. Same case happened in past during 2009 H1N1 influenza pandemic. Nothing was learnt from past.
In current scenario, it's need to grant compulsory license globally and at the same time also ensure the benefit of innovator companies because they also invested a huge amount of resources to invent new medicine. The WTO should come forward and make a path. So almost all countries, irrespective of financial status can get doses. Nothing is more important than human life, we have been already lost approx. 2 million people globally and it had also badly hit global economy as well. For what we are waiting???
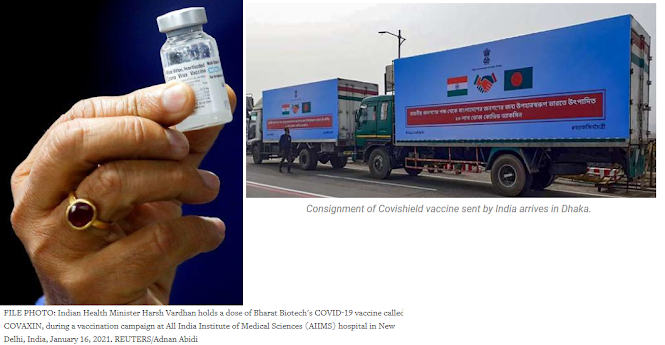
Fortunately, India is able to vaccinate its citizens and help other needy countries. India is the world's largest vaccine manufacturer, ahead of even china. United Nations Secretary-General Antonio Guterres termed the vaccine production capacity of India as the "best asset" the world has today. India has exported COVID-19 vaccines worth INR 338 crore till 8 Feb, said commerce and industry minister Piyush Goyal. The exports, which began in January, include grant of the vaccine doses to friendly countries and commercial shipments. India has become the fastest country in the world to reach the 4 million Covid-19 vaccination mark, achieving this feat in 18 days. Nearly 80 lakh beneficiaries have been vaccinated for COVID-19 across India in 28 days.
What can we do as an individual?
While the vaccine is an important part, we have to keep practice COVID Appropriate Behaviors (CAB).
Mask Use Handwashing 2 Gaj ki doori Prompt testing Prompt self isolation
About Author:
Author of this blog is Ravikant Ravi. Approx. 8 years of experience in Life Science domain . Post graduated from NIPER Mohali, India with specialization in Medicinal Chemistry.Contact: ravichemist28@gamil.com
References:
1) https://economictimes.indiatimes.com/markets/stocks/news/cadila-healthcare-gains-3-as-dcgi-plays-phase-iii-trials-of-covid-vaccine/articleshow/80091363.cms
2) https://www.bbc.com/news/world-asia-india-55748124
3) https://covid19.who.int/
4) https://covid19.who.int/region/searo/country/in
5) https://www.bharatbiotech.com/covaxin.html
6) https://www.mohfw.gov.in/pdf/COVID19VaccineOG111Chapter16.pdf
7) https://www.mohfw.gov.in/pdf/Covid19CommunicationStrategy2020.pdf
8) https://www.seruminstitute.com/product_covishield.php
9) https://www.businessinsider.in/india/news/aidan-asks-dcgi-to-withdraw-approval-for-bharat-biotechs-covaxin/articleshow/80078041.cms
10) https://www.icmr.gov.in/
11) https://msfaccess.org/india-and-south-africa-proposal-wto-waiver-ip-protections-covid-19-related-medical-technologies
12) https://msfaccess.org/wto-covid-19-trips-waiver-proposal-myths-realities-and-opportunity-governments-protect-access
13) https://www.daily-sun.com/post/534258/EU-Parliamentarians-Back-IndiaSouth-Africa-Covid-Vaccine-Patent-Proposal
14) https://www.politico.eu/article/europe-patent-grab-big-pharma/
15) https://www.wto.org/english/tratop_e/trips_e/public_health_faq_e.htm
16) https://www.gspkendra.com/2018/12/27/first-compulsory-license-in-india/
17) https://www.lifesciencesperspectives.com/2020/11/18/compulsory-patent-licensing-in-response-to-covid-19-recent-international-developments/
18) COVID-19, IP and access: Olga Gurula & Wen Hwa Lee
19)https://timesofindia.indiatimes.com/india/indias-vaccine-production-capacity-is-best-asset-world-has-today-says-un-chief/articleshow/80551643.cms
20)https://www.indiatvnews.com/news/india/global-media-praise-india-vaccine-diplomacy-covishield-covaxin-latest-news-684704
21)https://www.hindustantimes.com/india-news/india-vaccinates-close-to-8-million-beneficiaries-against-covid-19-in-28-days-101613230874577.html
22)https://www.oneindia.com/india/india-fastest-country-to-reach-4-million-covid-vaccination-mark-health-ministry-3211665.html








Very Informative work
ReplyDeleteThanks Rahul
DeleteCongratulations on sharing a super mindstroming article ! You have written an excellent article that should raise lots of eyebrows. Of course much of your subject was your own hard-won experience in the matter. Nevertheless, you must have done a wheelbarrow-full of research. I admire you. I guess this means we will be seeing more of you in the print media from now on. I say more power to you! Best wishes for you upcoming future.
ReplyDeleteSuper work Mr Ravi Ji, I have heard people complain that they did not get this or that opportunity or that someone else was just lucky. Well, the truth is that luck is only a small part of it. Because some people go through their life and create opportunities one of which is maybe going to be their key to success. Other people just sit on their sofa complaining that they never got a chance.
ReplyDeleteCongratulations for this insightful article Ravi it's very informative and covering important aspects in well explained way.
ReplyDeleteRavikant Sir !
ReplyDeleteNice article keep up growing.
Very nice bhaiya
ReplyDeleteVery Informative. Really appreciate your effort sir to provide this valuable content in regard COVID vaccine pipeline and licencing opportunity.
ReplyDeleteVery nice Ravi. Keep it up
ReplyDeleteVery precise and informative article about current pandemic situation and treatment available in the market
ReplyDeleteMany congratulations. Such an useful and insightful article. Thank you for sharing such an information. That would have taken much effort to collect and then collate in a single article. Much appreciated and honorable. Keep up sharing knowledge.
ReplyDeleteMany congratulations. Such an useful and insightful article. Thank you for sharing such an information. That would have taken much effort to collect and then collate in a single article. Much appreciated and honorable. Keep up sharing knowledge.
ReplyDeleteMany many congratulations ravi. Such an useful information and insightful article.keep it up
ReplyDeleteReally nice and informative article with true figure and facts..well done and keep it up...waiting for many more from your desk.
ReplyDelete